What Is Corrosion? Causes, Types, and Prevention Explained
Introduction
Corrosion is often described as the "natural enemy" of metal. It’s a gradual, destructive process that can compromise everything from pipelines and bridges to machines and lubricated systems. Whether you're an engineer, a maintenance professional, or just someone curious about material science, understanding corrosion is critical, especially in industries where reliability and safety are paramount. Corrosion mainly occurs in pipelines and metallic components exposed to harsh environments, often developing as a result of oxidation and prolonged contact with moisture, chemicals, or other corrosive agents. In this article, we’ll explore what corrosion is, its common causes and types, how it affects the lubrication industry, and the most effective ways to prevent it.
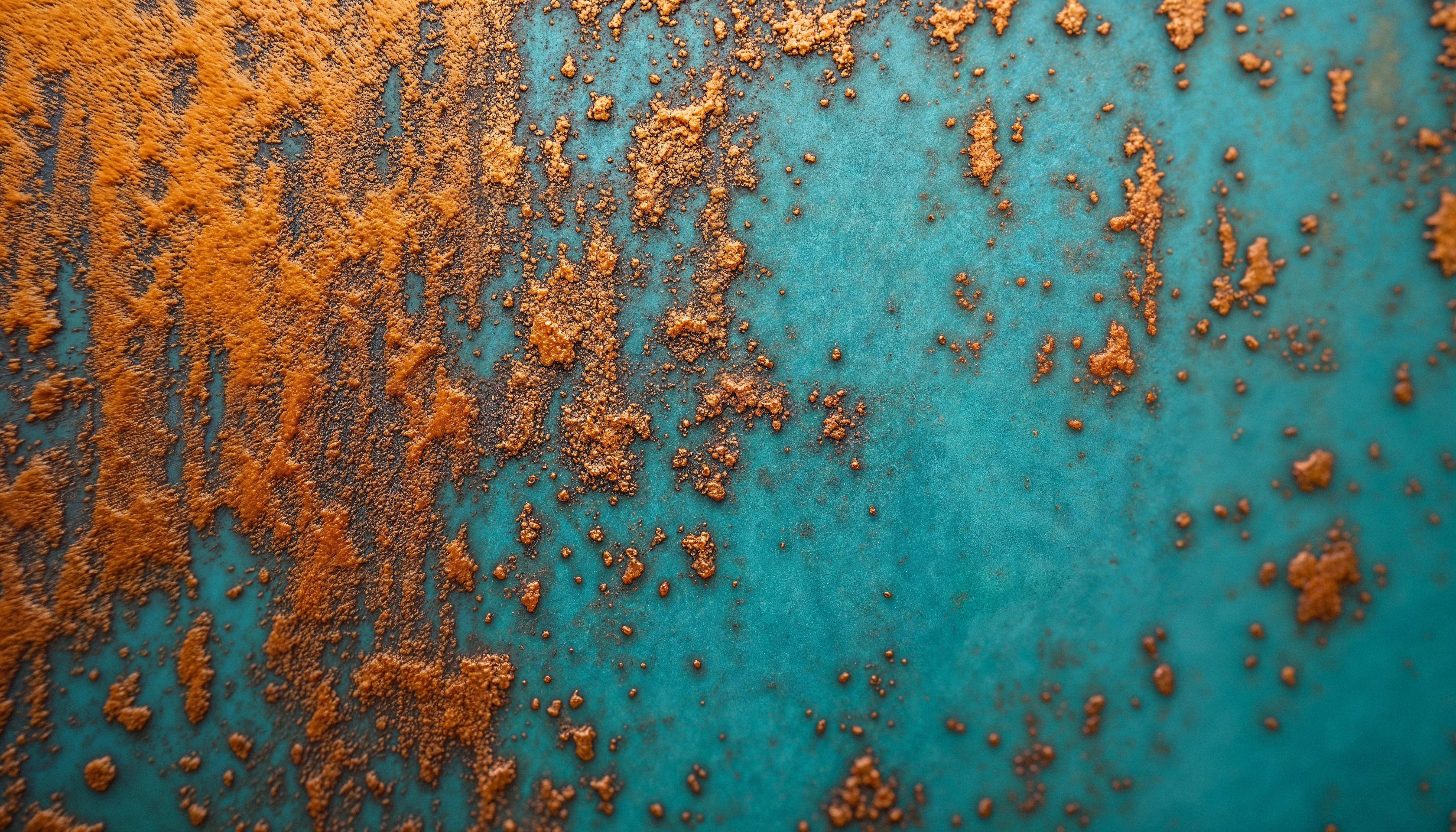
What Is Corrosion?
Corrosion is the chemical or electrochemical reaction between a
material (usually a metal) and its environment, which results in
the material's deterioration. Essentially, corrosion is nature’s
way of returning refined metals to their more stable, natural
states—typically oxides or sulphides.
The most well-known form of corrosion is rust, which occurs
when iron reacts with oxygen and moisture. But corrosion isn't
limited to just iron or steel; virtually all metals can corrode
under the right conditions.
For More Information About - Click Here.
Causes of Corrosion
Corrosion occurs due to several interrelated factors, including:
- Moisture and Oxygen: Water is a powerful catalyst for corrosion. When moisture and oxygen come into contact with metal, they initiate the electrochemical reactions that lead to oxidation.
- Electrochemical Reactions: Many forms of corrosion involve the flow of electric current. When two dissimilar metals are in contact in a conductive environment (like saltwater), a galvanic cell can form, causing one metal to corrode faster.
- Contaminants: Acids, salts, industrial pollutants, and even cleaning agents can accelerate corrosion by creating more aggressive environments.
- Temperature and Humidity: High temperatures and humidity levels increase the rate of corrosion, especially in enclosed or high-pressure systems like those in industrial machinery.
Types of Corrosion
Corrosion can manifest in several ways, depending on the material and environmental conditions. Key types include:
- Uniform Corrosion: This is the most common type, where the metal surface deteriorates evenly. Though slow, it can lead to significant material loss over time.
- Pitting Corrosion: Pitting creates small, deep holes in the metal surface and is particularly dangerous because it can be hard to detect until failure occurs.
- Galvanic Corrosion: Occurs when two dissimilar metals are in electrical contact in the presence of an electrolyte, leading to one metal corroding faster.
- Crevice Corrosion: Happens in confined spaces like under gaskets or fasteners where moisture can accumulate.
- Stress Corrosion Cracking (SCC): This is a result of tensile stress and corrosive environments causing unexpected fractures in metals, especially in high-stress industrial applications.
Corrosion in the Lubrication Industry
The lubrication industry faces unique challenges with corrosion, particularly in environments involving high heat, pressure, and reactive chemicals. While lubricants are primarily designed to reduce friction and wear, they also play a critical role in preventing corrosion.
1. How Corrosion Occurs in Lubricated Systems
- Water contamination in the lubricant (condensation, leaks, or ingress)
- Acid formation due to oxidation of the lubricant itself
- Metal-to-metal contact under boundary lubrication conditions
- Additive depletion, which weakens anti-corrosion protection over time
2. Effects of Corrosion on Lubrication Systems
- Surface pitting and scoring, reducing bearing life
- Blockages in filters or oil passages due to rust particles
- Increased wear and mechanical inefficiency
- Reduced equipment life and increased downtime
3. Anti-Corrosion Solutions in Lubrication
- Corrosion-inhibiting additives: Modern lubricants often include inhibitors that form a protective film on metal surfaces.
- Desiccant breathers and seals: To prevent moisture ingress.
- Regular oil analysis: Monitoring water content, pH, and additive levels can help predict corrosion risk.
- Synthetic lubricants: These often have better oxidation stability and moisture resistance.
How to Prevent Corrosion
Corrosion prevention involves a mix of design, material selection, protective coatings, and routine maintenance. Here are the most effective methods:
- Material Selection: Using corrosion-resistant alloys like stainless steel, titanium, or coated metals can significantly reduce corrosion risk.
- Protective Coatings: Paints, plating (e.g., zinc), and powder coatings create a barrier between the metal and the environment.
- Cathodic Protection: This method involves attaching a more reactive "sacrificial" metal that corrodes instead of the protected metal. Common in pipelines and marine structures.
- Environmental Control: Controlling humidity, temperature, and exposure to corrosive agents helps prolong equipment life. Desiccants and humidity indicators are often used in storage.
- Routine Inspection and Maintenance: Early detection is key. Regular visual inspections, oil analysis, and condition monitoring help catch corrosion before it becomes critical.
The Cost of Corrosion
Globally, corrosion costs economies trillions of dollars annually in repairs, maintenance, and downtime. In critical sectors like oil & gas, manufacturing, or transportation, a single corrosion-related failure can lead to catastrophic safety incidents and major financial loss. In the lubrication sector, poorly maintained lubricants or neglected corrosion can lead to:
- Premature machine failure
- Increased energy consumption
- Costly unplanned shutdowns
Conclusion
Corrosion is a silent but aggressive enemy of industrial and mechanical systems. While it’s a natural process, its effects can be minimized and even prevented with the right knowledge, practices, and materials.
Especially in the lubrication industry, where even small amounts of moisture or oxidized oil can initiate corrosion, proactive measures are essential. From choosing the right lubricant to implementing moisture control strategies and regular system monitoring, every step matters.
Understanding what corrosion is, how it works, and how to prevent it is the first step toward longer-lasting, safer, and more efficient systems.
Learn more about our services and industry insights by visiting our official LinkedIn page: Minimac Systems





